Nobel Prize for Chemistry 2022
What’s in News:
- The 2022 Nobel Prize in Chemistry has been awarded to chemists Carolyn R. Bertozzi and K. Barry Sharpless from the U.S., and Morten Meldal from Denmark, for their work in the field of click chemistry and bioorthogonal chemistry. In short it is a tool for building molecules.
- Dr. Sharpless was the first scientist to work on what is today called click chemistry – a branch of science that explores the assembly of molecules. He previously won the Chemistry Nobel in 2001.
- Dr. Meldal and Dr. Sharpless – independently of each other – presented the copper-catalysed azide-alkyne cycloaddition (CuAAC), a reaction that is now widely used in fields like medicinal chemistry.
- To simplify, Sharpless came up with the term ‘click chemistry’ and worked extensively on it, Meldal, independently of Sharpless, came up with a special chemical structure called ‘triazole’ which has many significant applications, and Bertozzi took the next step of developing click reactions that could work inside living organisms — ‘bioorthogonal’ reactions (a term she coined), take place living systems without interfering with native biochemical processes.
- The Nobel Prize in Chemistry 2022 is about making difficult processes easier. Click chemistry and bioorthogonal reactions have taken chemistry into the era of functionalism
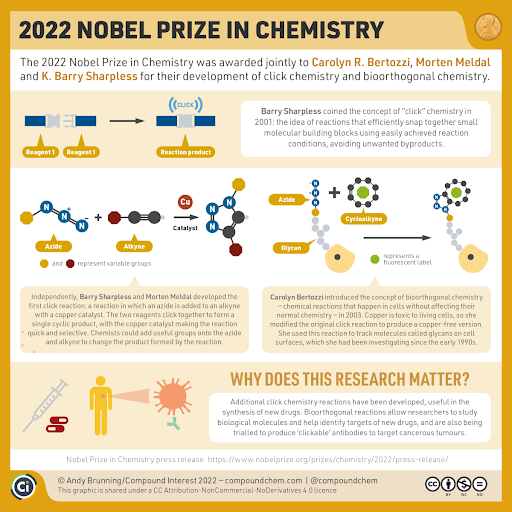
What is click chemistry?
- In simple terms, click chemistry is a functional field where molecules snap together quickly and efficiently – literally like a click.
- Chemists often try to recreate complex chemical molecules found in nature, and this has applications, among other things, in the field of medicine – how to target and block pathogens in cells. However, this process can be complicated and time-consuming.
- It is a set of powerful, highly reliable, and selective reactions for the rapid synthesis of useful new compounds and combinatorial libraries through heteroatom links.
- Instead of trying to wrangle reluctant carbon atoms into reacting with each other, Barry Sharpless proceeded to start with smaller molecules that already had a complete carbon frame. If chemists choose simple reactions – where there is a strong intrinsic drive for the molecules to bond together – they avoid many of the side reactions, with a minimal loss of material
- According to Dr. Sharpless, a reaction should be able to occur in the presence of oxygen and in water for it to be called that of click chemistry.
What is bioorthogonal chemistry?
- The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes.
The need for click chemistry
- Replicating reactions that involve bonds between carbon atoms – that are vital to the existence of life – is expensive and often leads to side reactions and loss of material.
- Instead of trying to make carbon atoms react with each other, Dr. Sharpless’s research focuses on using smaller molecules that already have a complete carbon frame.
- These molecules can further be linked using oxygen or nitrogen atoms as bridges. Simpler reactions, “where there is a strong intrinsic drive for the molecules to bond together”, may avoid the loss of material as well as the unwanted side reactions.
- Even if click chemistry is unable to provide exact replicas of natural molecules, it can help find molecules that fulfil the same purpose.
Building on the work by the other two winners
- Meldal through his experiments came up with the useful chemical structure called triazoles, which are stable and are found in pharmaceuticals, dyes and agricultural chemicals. He also found that the reaction he used could bind together numerous different molecules.
- Bertozzi, using the work of Sharpless and Meldal, came up with an efficient and innovative method to map glycans, which are carbohydrate-based polymers made by all living organisms. Her studies have led to the insight that some glycans appear to protect tumours from the body’s immune system, as they make the immune cells shut down. To block this protective mechanism, Bertozzi and her colleagues have created a new type of biological pharmaceutical. They have joined a glycan-specific antibody to enzymes that break down the glycans on the surface of the tumour cells. This pharmaceutical is now being tested in clinical trials on people with advanced cancer
Modern day applications
- Because of their high selectivity and specificity, click chemistry reactions find wide-range applications in areas like drug conjugation, materials science, etc.
- The bioorthogonal reactions are extremely significant in medicinal biochemistry since they do not interact with any other substances around them.
- Dr. Bertozzi has used bioorthogonal chemistry and her interpretation of click reactions to create a new pharmaceutical by joining a glycan-specific antibody to enzymes that break down the glycans on the surface of the tumour cells. This pharmaceutical is now being tested in clinical trials.
- Click chemistry solves problems of purification and separation of impure products in the synthesis of dendrimers, which are regularly-branched synthetic molecules that have applications in medicinal and materials chemistry.
- Click chemistry is utilised in the development of pharmaceuticals, for mapping DNA and creating materials that are more fit for purpose. Using bioorthogonal reactions, researchers have improved the targeting of cancer pharmaceuticals.
References:
- https://www.thehindu.com/sci-tech/science/chemistry-nobel-goes-to-trio-for-development-of-click-chemistry-and-bioorthogonal-chemistry/article65971551.ece
- https://www.thehindu.com/sci-tech/science/explained-research-in-click-and-bioorthogonal-chemistry-that-led-to-the-2022-nobel-prize-in-the-field/article65972562.ece
- https://indianexpress.com/article/explained/explained-sci-tech/nobel-prize-in-chemistry-announced-the-winners-work-its-significance-8191378/
Subscribe
Login
0 Comments
